Lycium barbarum pectin is a promising dietary component with potential gut health benefits, yet its precise structure–bioactivity relationship remains unclear. This study aimed to elucidate the unique architectural basis for its anti-inflammatory efficacy. Through an integrated approach combining an advanced structural analysis with in vivo and multiomics investigations, we report a novel pectin architecture. We identified a dual-configuration arabinogalactan-II (AG-II) that functions both as an autonomous domain and as a covalent side chain of rhamnogalacturonan-I (RG-I), forming a unique “glycan scaffold.” In a murine model of intestinal inflammation, the most effective pectin fraction (LBPA-III, 100 mg/kg) significantly alleviated tissue damage, restored the cytokine balance (e.g., TNF-α levels ↓ and IL-10 levels ↑), and inhibited neutrophil infiltration. Mechanistically, LBPA-III exerted its effects by remodeling the gut microbiota (e.g., reducing Helicobacteraceae abundance and increasing Muribaculaceae abundance), downregulating proinflammatory lipid metabolites (e.g., 1,2-dierucoyl-PE), and modulating the endocannabinoid system (CB2 levels ↑ and FAAH levels ↓). Our findings indicate that the synergistic interplay between AG-II and RG-I–AG-II results in the construction of a structural scaffold essential for the anti-inflammatory activity of L. barbarum pectin, positioning it as a candidate for targeted dietary interventions in the context of gut health.
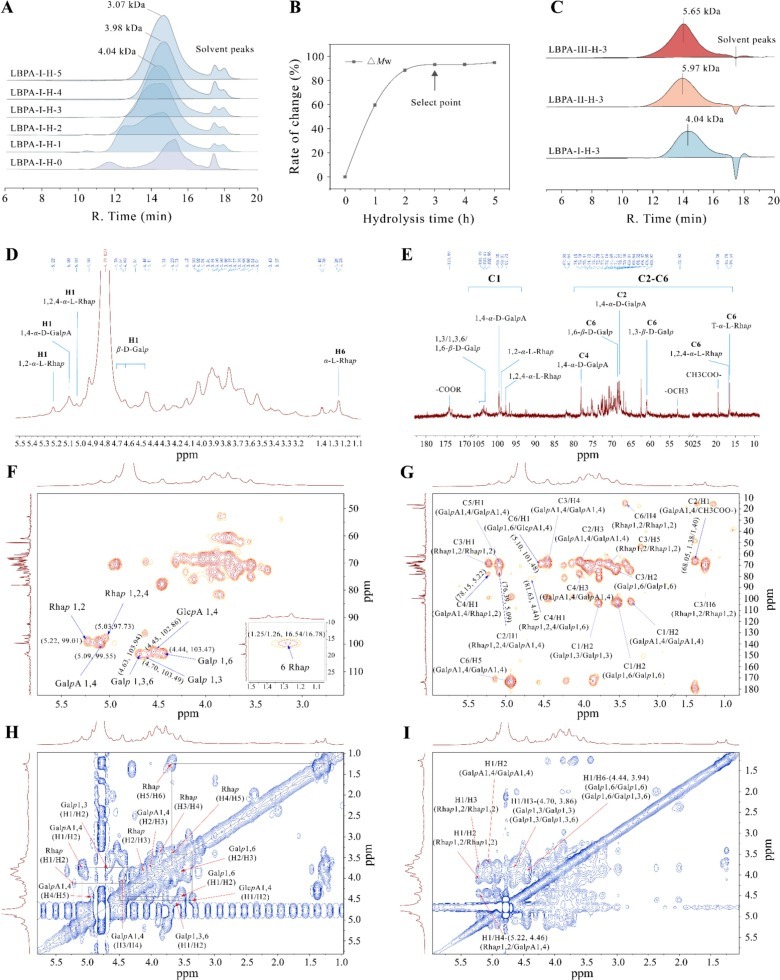
Fig. 5. Analysis of the core structures of LBPAs. (A) Distribution of the molecular weights of the fraction obtained from the dialysis bag at different times during LBPA-I hydrolysis. (B) Curves showing the rates of change in the molecular weight of LBPA-I at different hydrolysis times. (C) HPGPC spectra of LBPA-I-H-3, LBPA-II-H-3, and LBPA-III-H-3. (D, E) 1H and 13C NMR spectra of LBPA-III-H-3. (F) HSQC, (G) HMBC, (H) COSY, and (I) NOESY spectra of LBPA-III-H-3.
The link below will guide you to the reading:
https://doi.org/10.1016/j.carbpol.2026.124914
