Hypobaric hypoxia at high altitudes adversely affects the health and fertility of humans and animals globally. Previously, we showed that maternal hypoxia exposure led to granulosa cell (GC) autophagic cell death and oocyte maturation defects in female offspring. However, the impact of maternal hypoxia exposure on the reproduction and development of offspring in successive generations in mammals has yet to be fully elucidated. In this study, we exposed pregnant mice to hypoxia (F0) and examined the impact of maternal hypoxia exposure on male offspring in four generations (F1‒F4). The results revealed that maternal hypoxia mildly affected sperm DNA methylation in F1 males and caused severe developmental defects in F2 offspring in a male-biased manner. Notably, after mating F1 males with control females, a significant number of the male F2 fetuses were lost at embryonic day (E) 13.5 because of placental defects. Integrated analyses of RNA-seq and genome-wide DNA methylation (WGBS) data revealed that placentae from these male fetuses aberrantly expressed a list of imprinted genes, including Slc38a4, Jade1, Kcnq1 and Gnas, which are also differentially methylated in F1 sperm. These results indicated that maternal hypoxia altered the DNA methylation of imprinted genes in F1 sperm and affected the placental function and development of F2 males, leading to an unbalanced sex ratio. The findings of this study provide insight into the hypoxia environmental influences of the sex ratio and have important implications for deciphering hypoxia-induced reproductive impairments in mammals.
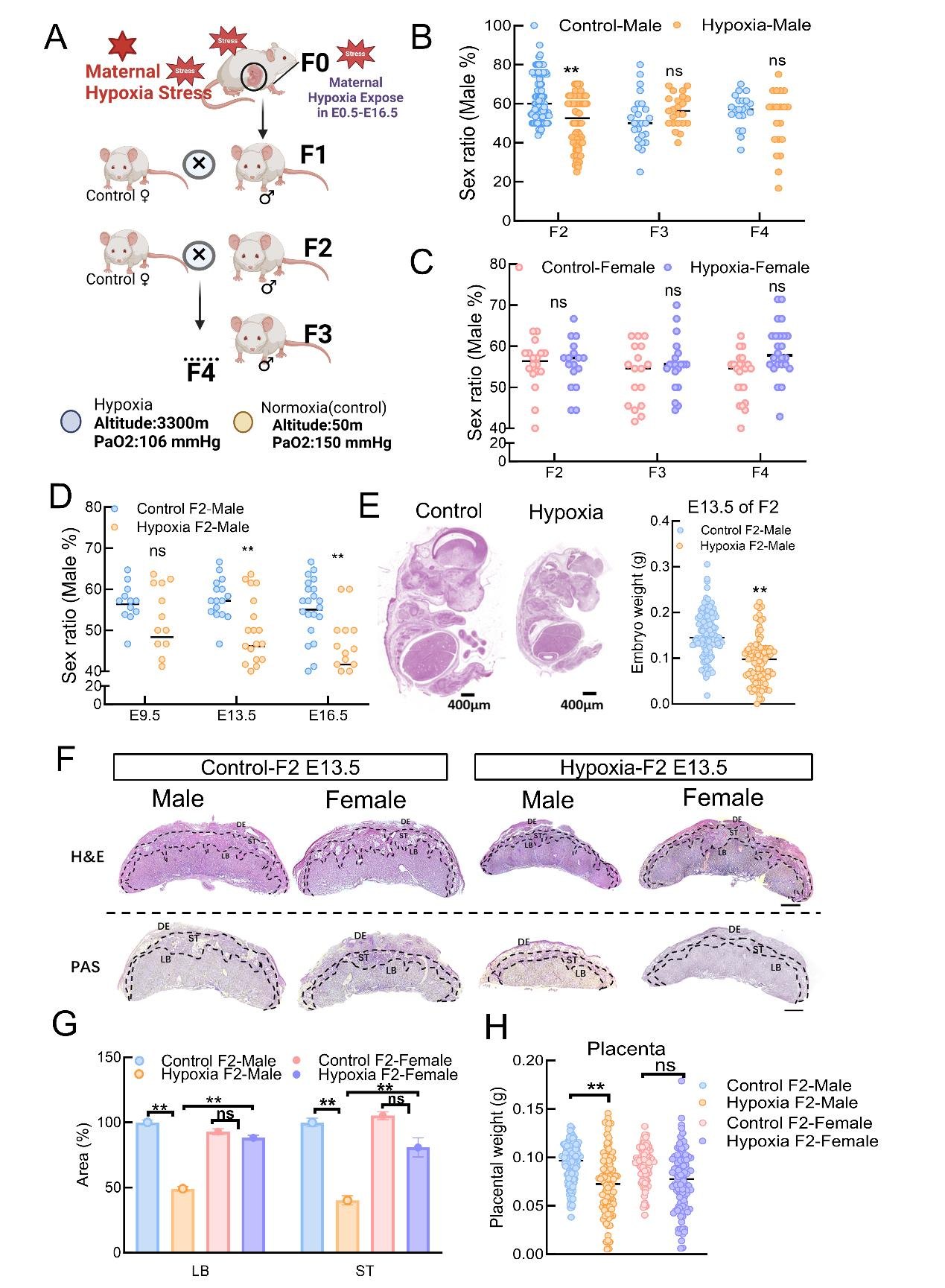
Figure 1Maternal hypoxia affects sex ratio of in an intergenerational manner A:Maternal hypoxia exposure in amale mouse model. B: Sex ratio (male%) in the control–male and hypoxia–malegroups in F2--F4. C:Sex ratio (male%) in the control and hypoxia exposed females (F2: control n=18, hypoxia: n=17; F3: control: n=18, hypoxia: n=20; F4: control: n=23, hypoxia: n=24). D:The percentage of embryonic sex ratio (percent of males) at E9.5, E13.5 and E16.5 (E9.5 Control-Male: n = 14, Hypoxia-Male: n = 16; E13.5 Control-Male: n = 18, Hypoxia-Male: n = 23; E16.5 Control-Male: n = 18, Hypoxia-Male: n = 26). E:Representative images of embryo morphology. The embryoswere prepared as paraffin slicesand stained with H&E. Scale bars: 400 μm. Embryo weights of E13.5 embryosharvested from control andF1 mice (control F1–male: n=112, F1–male: n=94). F:Histological images of H&E-stained and PAS-stained placentae at E13.5 in thecontrolF2-male, F2-male, control female and F2-female groups. DE, decidua; ST, spongiotrophoblast layer; LB, labyrinth layer. Scale bar: 400 μm. G:The percentagesof the areas of the LB and ST regions in the center of the placenta indicated bytheblack line were measured in three sections per placenta dissected from different groups (Control F2-Male: n = 6, Hypoxia F2-Male: n = 20, Control F2-Female: n = 5 and Hypoxia F2-Female: n = 16). H: Placental weight during embryonic development at E13.5. Note that the placental weight wassignificantly lower in maleembryos at E13.5 (Control F2-Male: n=113; F2-Male: n=95;Control F2-Female: n=103; F2-Female: n=102). The resultsare presented asthe means± SDs. All the experiments were repeated at least three times. ns:Notsignificant;*: P<0.05; **: P<0.01.
The link below will guide you to the reading:
https://www.zoores.ac.cn/article/doi/10.24272/j.issn.2095-8137.2025.031
