In the present study, six 7-O-glycosyl acylation of flavonoids derived from lutonarin and saponarin were obtained from barley seedlings by medium-pressure LC, high-speed counter-current chromatography and preparative HPLC guided by UPLC-QTOF-MS/MS. Their inhibitory effects on α-glucosidase were investigated. Structure–activity relationship showed 7-O-glycosyl acylation enhanced inhibitory effect on α-glucosidase with coumaroyl > feruloyl > hydroxybenzoyl > sinapoyl. The IC₅₀ values for saponarin and its four acylated products were 802.4 ± 0.48 μg/mL, 374.6 ± 0.16 μg/mL, 461.6 ± 0.27 μg/mL, 523.8 ± 0.24 μg/mL and 585.8 ± 0.42 μg/mL, respectively. Steady-state fluorescence spectroscopy revealed 7-O-glycosyl acylation enhanced the fluorescence quenching rate of α-glucosidase. Molecular docking revealed 7-O-glycosyl acylation formed more hydrogen bonds and hydrophobic interactions with α-glucosidase. Synchronous fluorescence spectroscopy showed all compounds altered the hydrophobic microenvironment surrounding the tryptophan residues in α-glucosidase. Circular dichroism spectroscopy indicated 7-O-glycosyl acylation modified the conformation and secondary structure of α-glucosidase.
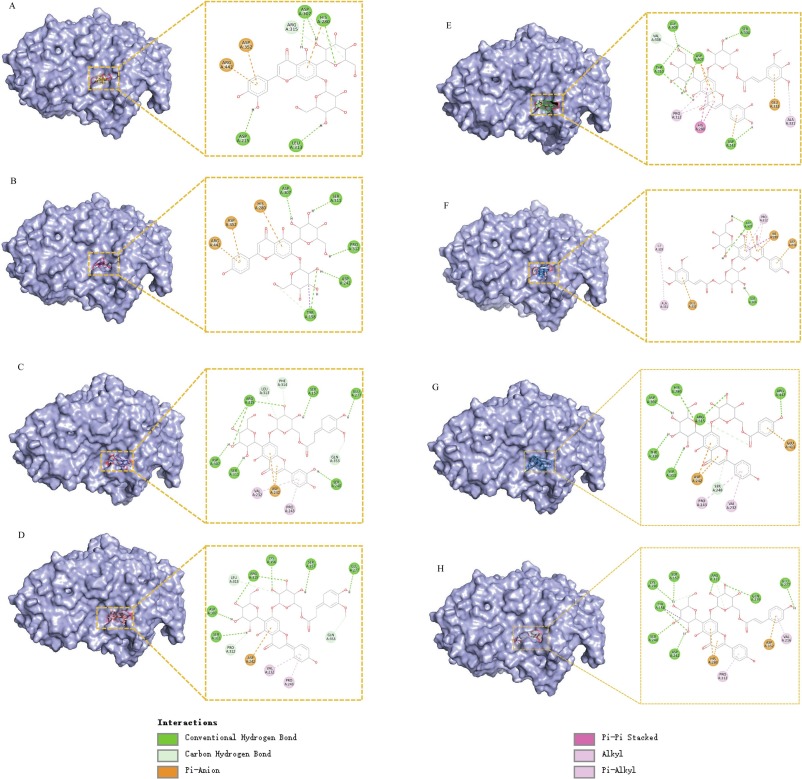
Fig. 6. Molecular docking results of flavonoid glycosides with α-glucosidase. Panels A-H represent the 3D binding conformations of compounds 1–5, 7, 8 and 9 with α-glucosidase, along with the 2D interaction diagrams of flavonoid glycosides with amino acid residues. The lower panel illustrates the different types of interactions formed between the compounds and amino acid residues.
The link below will guide you to the reading:
https://doi.org/10.1016/j.foodchem.2025.144891
