The hydrocarboxylation and hydrosilylation processes proposed in the copper-catalyzed reaction among carbon dioxide, diphenylacetylene and HSi(OEt)3 were comparatively studied with the aid of density functional theory calculations.
The study is to explore why the reaction preferred a hydrocarboxylation rather than a hydrosilylation process. It was found that theσ bond metathesis between Cu-C and H-Si involved in the hydrosilylation process had a significantly high reaction barrier in the presence of CO2 (47.4 kcal/mol). Instead, CO2 insertion and the subsequent σ bond metathesis between Cu-O and H-Si involved in the hydrocarboxylation process were confirmed kinetically feasible, consistent with the experimental facts.
Additional Information:
1 Author Information: Yi Zhao, Yuxia Liu, Siwei Bi, Yongjun Liu
Correspondence: siweibi@126.com (S. Bi), yongjunliu_1@sdu.edu.cn (Y. Liu).
2 Published: Journal of Organometallic Chemistry,2013, 745-746 :166-172
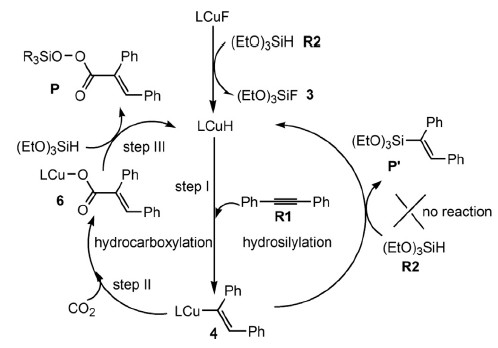
Scheme. Plausible catalytic cycle.
