Introduction – Stilbene glycosides are the primary constituents of Rheum tanguticum Maxim. ex Balf., to which different bioactivities has been attributed, including: anti-HIV, anti-oxidant, anti-tumour, anti-malarial, and anti-allergy activity.
However, effective methods for the isolation and purification of stilbene glycosides, such as trans-rhapontin, cis-rhapontin and trans-desoxyrhaponticin, from this herb are not currently available.
Objective – To develop an efficient method for the preparative isolation and purification of three stilbene glycosides from Rheum tanguticum Maxim. ex Balf. via high-speed counter-current chromatography (HSCCC).
Methods – A solvent system composed of chloroform:n-butanol:methanol:water (4:1:3:2, v/v/v/v) was developed for the separation. The upper phase was used as the stationary phase, and the lower phase was used as the mobile phase. The flow rate was 1.8 mL/min. The apparatus was controlled at 800 rpm and 25 oC, and the effluent was monitored at 280 nm. Chemical constituents were analysed by high-performance liquid chromatography (HPLC), and their structures were identified by 1 H- and 13C-NMR.
Results – Under the optimised conditions, 25.5mg trans-rhapontin, 16.0mg cis-rhapontin and 20.5mg trans-desoxyrhaponticin were separated from 80mg crude sample; the isolates had purities of 99.6, 97.2 and 99.2%, respectively.
Conclusion – A simple and efficient HSCCC method has been optimised for the preparative separation of stilbene glycosides from Rheum tanguticum Maxim. ex Balf.
However, effective methods for the isolation and purification of stilbene glycosides, such as trans-rhapontin, cis-rhapontin and trans-desoxyrhaponticin, from this herb are not currently available.
Objective – To develop an efficient method for the preparative isolation and purification of three stilbene glycosides from Rheum tanguticum Maxim. ex Balf. via high-speed counter-current chromatography (HSCCC).
Methods – A solvent system composed of chloroform:n-butanol:methanol:water (4:1:3:2, v/v/v/v) was developed for the separation. The upper phase was used as the stationary phase, and the lower phase was used as the mobile phase. The flow rate was 1.8 mL/min. The apparatus was controlled at 800 rpm and 25 oC, and the effluent was monitored at 280 nm. Chemical constituents were analysed by high-performance liquid chromatography (HPLC), and their structures were identified by 1 H- and 13C-NMR.
Results – Under the optimised conditions, 25.5mg trans-rhapontin, 16.0mg cis-rhapontin and 20.5mg trans-desoxyrhaponticin were separated from 80mg crude sample; the isolates had purities of 99.6, 97.2 and 99.2%, respectively.
Conclusion – A simple and efficient HSCCC method has been optimised for the preparative separation of stilbene glycosides from Rheum tanguticum Maxim. ex Balf.
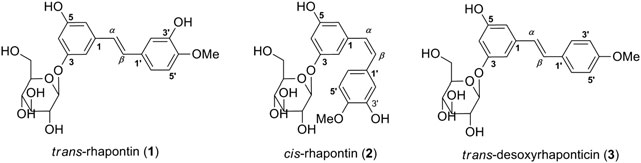
Figure 1. The chemical structures of trans-rhapontin (1), cis-rhapontin (2) and trans-desoxyrhaponticin (3).
Additional Information:
1. Author Information:Xiaohui Zhao,Fa Han,Yulin Li,Huilan Yue
Correspondence: xhzhao@nwipb.ac.cn
2. Publication History: Phytochem. Anal. 2013, 24, 171–175
1. Author Information:Xiaohui Zhao,Fa Han,Yulin Li,Huilan Yue
Correspondence: xhzhao@nwipb.ac.cn
2. Publication History: Phytochem. Anal. 2013, 24, 171–175
