Bacteroides thetaiotaomicron α-glucosidase BtGH97a is an inverting enzyme. In this paper, the hydrolysis mechanism of p-nitro-phenyl α-D-glucopyranoside (pNP-Glc) catalyzed by BtGH97a was firstly studied by using quantum mechanical/molecular mechanical (QM/MM) approach. Two possible reaction pathways were considered. In the first pathway, a water molecule deprotonated by a nucleophilic base (here E439 or E508) attacks firstly on the anomeric carbon of pNP-Glc, then a proton from an acid residue (E532) attacks on the glycosidic oxygen to finish the hydrolysis reaction (named as nucleophilic attack-first pathway). In the second pathway, the proton from E532 attacks firstly on the glycosidic oxygen, then the water deprotonated by the nucleophilic base attacks on the anomeric carbon of pNP-Glc (named as proton attack-first pathway). Our calculation results indicate that the nucleophilic attack-first pathway is favorable in energy, in which the nucleophilic attack process is the rate-determining step with an energy barrier of 15.4 kcal/mol in the case of residue E508 as nucleophilic base. In this rate-determining step, the deprotonation of water and the attack on the anomeric carbon are concerted. In the proton attack-first pathway, the proton attack on the glycosidic oxygen is the rate-determining step, and the energy barrier is 24.1 kcal/mol. We conclude that the hydrolysis mechanism would follow nucleophilic attack-first pathway.
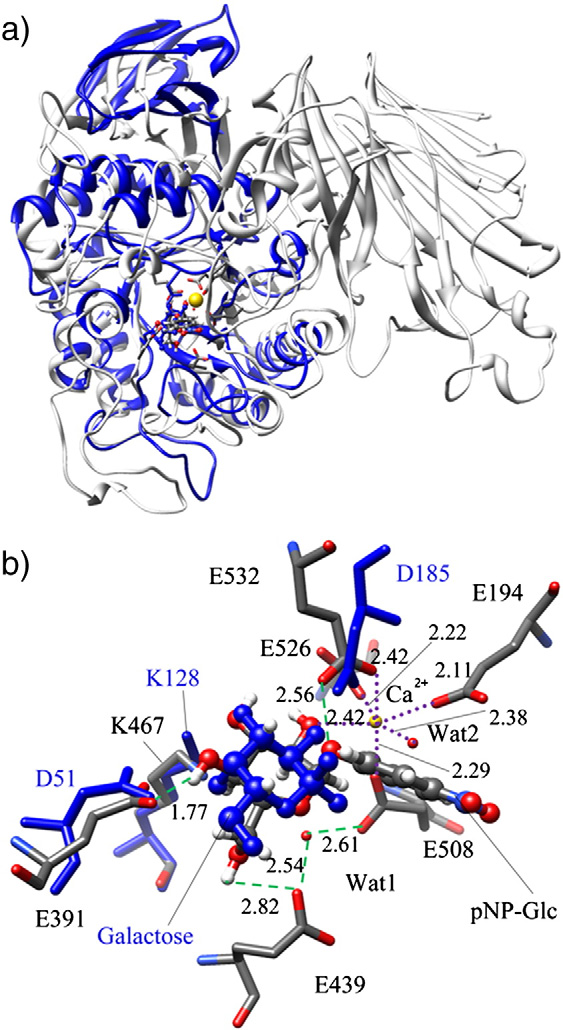
Fig. (a) Superposition of the docking structure of BtGH97a (gray, PDB ID: 2JKE) with
the closest GH27 α-galactosidase (blue, PDB ID: 1UAS); (b) pocket residues of the superposition.
(For interpretation of the references to color in this figure legend, the
reader is referred to the web version of this article.)
Additional Information:
1. Author Information: Jinhu Wang, Xiang Sheng, Yi Zhao, Yongjun Liu, Chengbu Liu
Correspondence:yongjunliu_1@sdu.edu.cn
2. Published : Biochimica et Biophysica Acta 1824 (2012) 750–758
